Reprocessing of spent nuclear fuel (Sustainability Assessment)
This page refers to "Safety of NFCFs".
In this section, firstly, a short description of the main processes in a reprocessing facility is given and the corresponding specific safety issues are discussed. Secondly, the assessment method will be discussed based on the corresponding criteria of the INPRO methodology in the area of safety, which have been, where necessary, adapted to the specific issues of this type of facility. Fuel reprocessing is the technology for recovery and recycle of nuclear fuel including bred fissile material from spent fuel assemblies (or fuel elements or bundles) discharged from a nuclear reactor after irradiation. As of beginning of 2017 the following five countries have commercial reprocessing facilities in operation: France (La Hague), Great Britain (Sellafield), India (Trombay, Tarapur, and Kalpakkam), Japan (Rokkasho, Tokai), and the Russian Federation (Tscheljablinsk). These facilities all use variants of the so-called PUREX process to be briefly described in the following. In recognition of the importance of spent fuel reprocessing in the back end of the fuel cycle, the IAEA has provided a forum for exchange of information on the status and trends in spent fuel reprocessing since the 1970s, from which several publications have been issued [1][2][3][4] (see also Refs[5][6][7]). Spent nuclear fuel transportation has not been considered in this manual as an independent stage of nuclear fuel cycle. INPRO methodology implies that safety of spent fuel transportation is to be considered as part of the INPRO assessment either of the spent fuel storage facility or the spent fuel reprocessing facility.
Contents
Purex process
Historically, several extraction systems were explored for reprocessing of nuclear fuel, before an efficient method was identified. The combination known generically as PUREX (plutonium uranium redox extraction), which utilizes as extractant tri-butyl phosphate (TBP) mixed in a largely inert hydrocarbon diluent, has now replaced all earlier solvent extraction media. The PUREX process has a number of advantages in comparison to earlier versions including lower solvent volatility and flammability, higher chemical and radiation stability of the solvent and lower operating costs. Since the opening of the first PUREX plant at Savannah River in 1954, the PUREX process has been utilized in a variety of flow sheets, and, as stated before, is still being used in all commercial reprocessing plants currently operating.
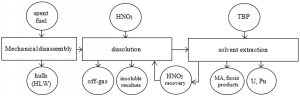
Commercial reprocessing relies on a series of four main technological operations: fuel handling and shearing, fuel dissolution, materials separation and purification, and finally, waste treatment and conditioning. The aqueous process route is assumed to be viable up to a burn-up of at least 100 GWd/t; radiation and chemical degradation of the extractant being the limitation for processing fuels of higher burn-up. A typical schematic of a conventional PUREX process is shown in Fig. 1.
The major steps in the PUREX process are:
- Head end process: the fuel is received, chopped and prepared for dissolution;
- The U, Pu, minor actinides and fission products go into solution in nitric acid;
- The hull is monitored for residual fissile material content and treated as solid waste;
- Off gas is treated to remove radioactive isotopes such as iodine, krypton and xenon:
- Separation of U and Pu from fission products and minor actinides, using aqueous organic solvents. In the PUREX process, aqueous phase is nitric acid and organic phase is TBP in hydro-carbon solution. Aqueous/ organic phase contact is achieved by centrifugal or other types of contactors such as pulsed columns and mixer-settlers;
- Purification and concentration of U and Pu and storage; and
- Recovery of minor actinides and long lived fission products to be managed as high level waste.
Other reprocessing methods
There is a variety of other reprocessing methods under different state of development. They can be grouped into the following categories[4]:
- Evolutionary technologies based on aqueous separation methods using TBP as extractant (derived from the PUREX process). Examples are: COEX (France), NUEX (UK), Simplified PUREX and REPA (Russian Federation), THOREX (India), NEXT (Japan);
- Innovative aqueous processes using new extractants. Examples are: DIAMEX-SANEX and GANEX (France), UREX+3a and UREX+1a (USA), and PARC and ARTIST (Japan);
- Non aqueous technologies (dry route) – pyro chemical processes. Examples are: Pyro-chemical-(liq-liq) process (France), DDP (Russian Federation), Electro Metallurgical process (USA);
- Hybrid methods combining hydro and pyro chemical processes. Examples are: FLUOREX (Japan), Combined process including gas fluoride and extraction technologies (Russian Federation); and
- Innovative processes using fluid extraction or precipitation methods. Examples are: Fluid extraction (Russian Federation, Japan), Ion-exchange processes (Japan, Belgium), Sedimentation (Japan).
Safety issues in a reprocessing facility
Among the NFCFs other than nuclear reactors, reprocessing facilities are the most complex with respect to safety. The potential safety hazards to which fuel reprocessing facilities are prone include criticality excursions, radiation exposure, chemical reactions, fire and explosion.
Safety issues related to the storage of spent nuclear fuel at a reprocessing facility before the reprocessing starts are discussed in the following Section.
Criticality
Criticality control is a dominant safety issue for reprocessing plants due to the large amount of fissile materials treated and the presence of water, a moderator, in many parts of the plant. To prevent criticality relevant parameters such as the mass, volume, concentration of fissile material have to be controlled and geometry of components that contain fissile material has to be designed accordingly.
The following areas in a reprocessing facility have a significant risk of criticality[8]: Shear pack (due to accumulation of spent fuel powder), storage of hulls (due to residual fuel), dissolver, solvent extraction process, and purification of U and Pu, and conversion to oxide. Use of neutron poisons (in liquids or in casings of equipment) can allow larger sized components and reduce the possibility of criticality.
Radiation exposure
Dispersion of (highly) radioactive material may result in exposure of personnel. Thus, several barriers are necessary to be installed to avoid dispersion leading to contact of radioactive material with the workers. Two types of containment are used as barriers against dispersion: static and dynamic containments. The first static containment (or barrier) is composed of the walls of process equipment, i.e. such as tanks and pipes. Almost all process equipment is contained within (hot) cells. Thus, the second one consists of the enclosures of the (hot) cells, i.e. typically thick concrete walls. The third containment is formed of the external walls of the building. An additional dynamic containment function is achieved by adequate ventilation of the process equipment, vessels and cells, plus the working areas. The IAEA safety standard[9] states:
“4.28. In a reprocessing facility (for most areas), three barriers (or more, as required by the safety analysis) should be provided, in accordance with a graded approach. The first static barrier normally consists of process equipment, vessels and pipes, or gloveboxes. The second static barrier normally consists of cells around process equipment or, when gloveboxes are the first containment barrier, the rooms around the glovebox(es). The final static barrier is the building itself. The design of the static containment system should take into account openings between different confinement zones (e.g. doors, mechanisms, instruments and pipe penetrations)”.
To minimize external radiation exposure, the working areas need to be far away from radioactive material, adequate shielding can be established adapted to nature and energy of the radiation, and the exposure time of workers needs to be limited.
Radiation exposure of the public and the environment is discussed in the INPRO methodology manual on environmental impact of stressors[10].
Fire and explosion
A large fire spreading through a reprocessing plant or an explosion might be one of the main causes of dispersion of radioactive material into the environment, notably in the event of ventilation system failure, and damage to engineered controls. Reprocessing plants use flammable solvents (e.g. kerosene or dodecane), certain pyrophoric materials (e.g. zirconium powder, TBP), and agents (e.g. hydrogen, hydrazine) with oxidising and reducing properties. For example, the fragmentation of LWR fuel assemblies by shearing during head-end processing produces powder of Zircaloy that present a potential for ignition or explosion. Hydrogen gas probably represents the greatest potential cause of explosion, due to its rapid rate of diffusion, its low ignition energy input, and the wide range of concentration limits that may give rise to an explosion.
The risk of fire can be reduced by eliminating sources of ignition and hot points and by installing fire detection and extinction systems.
In the following an example is presented of an explosion in a reprocessing facility, namely, the so-called ‘red oil’ explosion. Red oil is the name of a substance of non-specific composition formed when an organic phase consisting of tri-butyl-phosphate (TBP) and diluents in contact with concentrated nitric acid is heated above 120°C under reflux. At temperatures above 130°C, the degradation of TBP, diluents, and nitric acid proceeds at rates fast enough to generate heat and voluminous amounts of detonable vapour. The generated heat further increases the temperature of the liquid, which in turn increases the rate of reaction (i.e. a runaway or autocatalytic reaction). For a comprehensive review, see Refs[11][12][13].
Three red oil events have occurred in the U.S. Department of Energy’s defence nuclear facilities complex at the Hanford Site in 1953, and at the Savannah River Site in 1953 and 1975[14][15]. A red oil explosion also occurred in 1993 at the Tomsk-7 facility in Seversk, Russian Federation. More recent fire and explosion incident occurred in 1997 at the Bituminization Demonstration Facility of the Tokai reprocessing plant in Japan.
A report by U.S. Defence Nuclear Facilities Safety Board highlights the conditions under which red oil formation and explosion can take place and means to avoid such incidents[16].
Containment of radioactive material and/or toxic chemicals
Due to the use of highly aggressive acids and other chemicals in a reprocessing facility corrosion in process equipment can cause leaks of radioactive material and/or toxic chemicals.
In the following an example is presented of a leakage of radioactive material that occurred on 21 April 2005 in the feed clarification cell of the THORP plant at Sellafield in Great Britain. The THORP plant is designed to reprocess irradiated fuels produced by advanced gas-cooled reactors (AGR) and light water reactors. Reprocessing campaigns have been carried out with uranium oxide spent fuel originally enriched in 235U by up to 4.8 %. The plant had already reprocessed around 5700 tonnes since its commissioning in 1994. During the incident about 83 m3 of clarified radioactive fluid leaked into one of the recovery pans and was discovered during a camera inspection of the main feed clarification cell. This cell is closed off to personnel at all times and its walls guarantee the radiological protection of the adjacent premises. The toxic fluid present in the recovery pan contained uranium and plutonium that were yet to be separated from the fission products and estimated to represent about 20 tonnes and 200 kg, respectively. The plant was shut down as soon as the incident was discovered. The main cause of the leakage was from a failed pipe running between two accountancy tanks. Only the first barrier consisting of the transfer pipe failed during this incident. The static and dynamic integrity of the two remaining containment barriers remained intact. The operator emphasized that the leak posed no danger to the workers or the environment. In particular, no abnormal activity around the plant’s stack has been detected. The operator also underlined the absence of any risk of criticality, which was corroborated by the British safety regulator.
A leak in the pipes and tanks of reprocessing facilities, if undetected, can create a hazard of internal flooding. Examples of fluids causing flooding are: cooling water, heating water, treated water, chemical solutions, fire-fighting water, etc. In a reprocessing plant, the most important hazardous consequences generated by flooding are: criticality, damage to equipment fulfilling safety functions, and dispersion into the environment of radioactive material transported by the fluid involved.
To prevent a release of radioactive material and/or toxic chemical to the outside of the plant several barriers (combinations of static and dynamic containments) are necessary: The first barrier is the casing of the equipment (e.g. wall of a vessel or pipe), the second barrier is a glove box or hot cell, and the last one is the building of the facility. Additionally, dynamic containments are to be provided by ventilation systems in process equipment, hot cells and glove boxes but also in the working area of the facility.
External hazards
Reprocessing facilities are expected to be designed against all credible external hazards (see Section 2.1 and 2.6 of NFCF). The IAEA Safety Standard[17] provides a list of selected external postulated initiating events including natural phenomena and human induced phenomena:
“(a) Earthquakes, volcanoes and surface faulting;
(b) Meteorological events, including extreme values of meteorological phenomena and rare events such as lightning, tornadoes and tropical cyclones;
(c) Floods, including water waves induced by earthquakes or other geological phenomena or floods and waves caused by failure of water control structures;
(d) Geotechnical hazards, including slope instability, collapse, subsidence or uplift of the site surface, and soil liquefaction;
(e) External human induced events, including transport events such as aircraft crashes and accidents at surrounding activities such as chemical explosions”.
See also
- NFCF
- Mining and milling of uranium and thorium
- Uranium refining/conversion and enrichment
- Uranium oxide and MOX fuel fabrication
- Storage of spent nuclear fuel
[ + ] Assessment Methodology | |||||
|---|---|---|---|---|---|
|
|||||
References
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, Implications of Partitioning and Transmutation in Radioactive Waste Management, IAEA Technical Report Series No. 435, IAEA, Vienna (2004).
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, Status and Trends in Spent Fuel Reprocessing, IAEA-TECDOC-1103, IAEA, Vienna (1999).
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, Status and Trends in Spent Fuel Reprocessing, IAEA-TECDOC-1467, IAEA, Vienna (2005).
- ↑ 4.0 4.1 INTERNATIONAL ATOMIC ENERGY AGENCY, Spent Fuel Reprocessing Options, IAEA-TECDOC-1587, Vienna (2008).
- ↑ LONG, J., Engineering for Nuclear Fuel Reprocessing, Gordon and Breach Publishers, New York, (1967).
- ↑ SCHULZ, W., BURGER, L., NAVARATIL, J., BENDER, K., Science and Technology of TBP Volume III, Applications of Tri-Butyl Phosphate in Nuclear Fuel Reprocessing, CRC Press, Florida (1984).
- ↑ SKIBA, O., IVANOV, V., The State and Prospects of the Fuel Cycle Development Using Pyro-electrochemical Processing in Molten Salts, Molten Salts in Nuclear Technologies Seminar, Dimitrovgrad, June 19-22, (1995).
- ↑ OECD/NUCLEAR ENERGY AGENCY (NEA), The Safety of the Nuclear Fuel Cycle, Third Edition, NEA No.3588, OECD/NEA, Paris (2005).
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, Safety of Nuclear Fuel Reprocessing Facilities, IAEA Safety Standards, Specific Safety Guide No. SSG-42, IAEA, Vienna (2017).
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, INPRO Methodology for Sustainability Assessment of Nuclear Energy Systems: Environmental Impact of Stressors, IAEA Nuclear Energy Series No. NG-T-3.15, IAEA, Vienna (2016).
- ↑ PADDLEFORD, D., FAUSKE, H., Safe Venting of Red Oil Runaway Reactions, Report WSRC-MS-94-0649, US-DOE, Washington (1994).
- ↑ RUDISILL, T., CROOKS, W., Initiation Temperature of Runaway TBP/HNO3 Reactions, Report WSRC-TR-2000-00427, US-DOE, Washington (2000).
- ↑ JAMES, N., SHEPPARD, G., Red-oil Hazards in Nuclear Fuel Reprocessing, Nuclear Engineering and Design, Vol. 130, issue 1, Elsevier (1991).
- ↑ VANDERCOOK, R., Summary of Red Oil Issues at Hanford, WHC-WM-TI-466, Westinghouse Hanford Company (1991).
- ↑ WATKIN, J., GORDON, P., LAGNEW, S., “Red Oil” Safety Evaluation Project, Briefing presented to the Defence Nuclear Facilities Safety Board, USA, Washington (1993).
- ↑ DEPARTMENT OF ENERGY, Control of Red Oil Explosions in Defence Nuclear Facilities, Technical report DNFSB/TECH-33, Defence Nuclear Facilities Safety Board, USA, Washington (2003). [1]
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, Safety of Nuclear Fuel Cycle Facilities, IAEA Safety Standards, Specific Safety Requirements No. SSR-4, IAEA, Vienna (2017).