Uranium refining/conversion and enrichment (Sustainability Assessment)
This page refers to "Safety of NFCFs".
In this section, firstly, a short description of the main processes in U refining/ conversion and uranium enrichment facilities is given and the corresponding specific safety issues are discussed. Secondly, the assessment method is presented based on the corresponding criteria of the INPRO methodology in the area of safety, which have been, where necessary, adapted to the specific issues of these two kinds of NFCFs.
As of end of 2016 the following countries have uranium refining/ conversion facilities in operation: Argentina (Pilcaniyeu), Canada (Port Hope), China (Lanzhou), France (Malvesi, Pierrelatte), Iran (Isfahan), Russian Federation (Glazov; Seversk, Angarsk), USA (Metropolis).
Countries with uranium enrichment facilities operating (or under construction) at the end of 2016 are: Argentina (Pilcaniyeu), Brazil (Resende), China (Lanzhou, Hanzhong), France (Tricastin), Germany (Gronau), India (Karnataka), Iran (Natanz, Qom), Japan (Rokkasho), Netherlands (Almelo), Pakistan (Kahura), Russian Federation (Novouralsk, Seversk, Zelenogorsk, Angarsk), United Kingdom (Capenhurst), USA (Lea County).
Contents
Uranium refining and conversion to hexafluoride UF6
The end product from the mining and milling stage of the fuel cycle is called ‘yellow cake’, which is essentially an impure uranium compound.
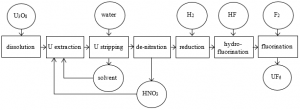
Refining or purification processes are required to bring the uranium to the standard of purity necessary for nuclear reactor fuel element manufacture. The various stages in the purification process are dissolution, solvent extraction, concentration and thermal de-nitration to uranium trioxide (Fig.1). The processes are typical for those of a chemical industry handling a chemically toxic rather than a radioactive material, the toxicity of natural uranium being about the same as that of lead.
For the conversion process, i.e. production of uranium hexafluoride, there are several methods: a batch process using chlorine tri-fluoride; direct fluorination; and a modern method using pure uranium tri-oxide, UO3, which is converted to UF4, uranium tetra-fluoride, then to uranium hexafluoride, UF6, prior to enrichment. The chemical reactions[1] are:
The reactions are generally carried out using fluidized bed technology. A typical flowchart of uranium refining and conversion is shown in Fig. 1.
For details on the conversion processes see Ref[2].
Uranium enrichment
Natural uranium primarily contains two isotopes, 238U (99.3 %) and 235U (0.7 %). The concentration of 235U, the fissionable isotope in uranium, needs to be increased to 3 to 5 % for use as a nuclear fuel in LWRs.
The uranium enrichment can be performed in several ways:
- Electromagnetic isotope separation (EMIS);
- Thermal diffusion;
- Aerodynamic uranium enrichment process;
- Chemical exchange isotope separation;
- Ion exchange process;
- Plasma separation process;
- Gaseous diffusion process;
- Gas centrifuge process, and
- Laser isotope separation.
Of these, gaseous diffusion processes and gas centrifuge processes are currently used in commercial industries . However, no new diffusion plants are being built because the centrifuge plants are more economical. For details on the enrichment processes see Refs[3][4][5].
Gaseous diffusion process
Uranium arrives at the plant in the form of solid UF6. It is vaporized and advantage is taken of the difference in the molar masses of the isotopes to separate them selectively by passage of UF6 through a porous wall, a ‘barrier’. The lighter isotope 235U passes more easily through the wall than 238U. Because enrichment by means of a single barrier is very small, it is necessary to repeat the operation a great number of times. The elementary unit in enrichment is the stage, which is composed of a diffuser containing barriers; a compressor which forces the UF6 to pass through the barriers and an exchanger, which removes the heat generated by the compressor. The stages are placed in a series. The part of the flux that passes through the barrier goes to the following stage; the part that does not pass is directed towards the lower stage. The stages are linked together of ten to twenty units that constitute a group. Several groups constitute a cascade. UF6 is introduced into the centre of the cascade. The UF6 that has been enriched in uranium 235U is withdrawn at one end and the depleted UF6 at the other. One of the disadvantages in gaseous diffusion process is the heavy use of electricity.
Gas centrifuge process
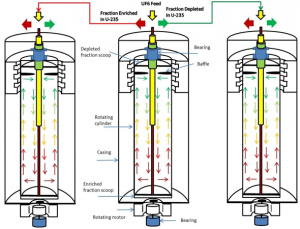
In the gas centrifuge uranium-enrichment process, gaseous UF6 is fed into a cylindrical rotor that spins at high speed inside an evacuated casing. Because the rotor spins so rapidly, centrifugal force results in the gas occupying only a thin layer next to the rotor wall, with the gas moving at approximately the speed of the wall. Centrifugal force also causes the heavier 238UF6 molecules to tend to move closer to the wall than the lighter 235UF6 molecules, thus partially separating the uranium isotopes. This separation is increased by a relatively slow axial counter-current flow of gas within the centrifuge that concentrates enriched gas at one end and depleted gas at the other. This flow can be driven mechanically by scoops and baffles or thermally by heating one of the end caps. A schematic diagram of the gas centrifuge is shown in Fig. 2.
The main subsystems of the centrifuge are rotor and end caps; top and bottom bearing/suspension system; electric motor and power supply (frequency changer); centre post, scoops and baffles; vacuum system; and casing. Because of the corrosive nature of UF6, all components that come in direct contact with UF6 need to be fabricated from, or lined with, corrosion-resistant materials. The separative capacity of a single centrifuge increases with the length of the rotor and the rotor wall speed. The primary limitation on rotor wall speed is the strength-to-weight ratio of the rotor material. Another limitation on rotor speed is the lifetime of the bearings at either end of the rotor. Balancing of rotors to minimize their vibrations is especially critical to avoid early failure of the bearing and suspension systems. Because perfect balancing is not possible, the suspension system needs to be capable of damping some amount of vibration.
One of the key components of a gas centrifuge enrichment plant is the power supply (frequency converter) for the gas centrifuge machines. The power supply is supposed to accept alternating current (AC) input at the 50 or 60 Hz line frequency available from the electric power grid and provide an AC output at a much higher frequency (typically 600 Hz or more). The high-frequency output from the frequency changer is fed to the high-speed gas centrifuge drive motors (the speed of an AC motor is proportional to the frequency of the supplied current). The centrifuge power supplies are expected to operate at high efficiency, provide low harmonic distortion, and provide precise control of the output frequency.
The casing is needed both to maintain a vacuum and to contain the rapidly spinning components in the event of a failure. If the shrapnel from a single centrifuge failure is not contained, a ‘domino effect’ may result and destroy adjacent centrifuges. A single casing may enclose one or several rotors. The enrichment effect of a single centrifuge is small, so they are linked together by pipes into cascades, to obtain the required enrichment. Once started, a modern centrifuge runs for more than 10 years with no maintenance.
Enrichment of UF6 resulting from uranium recovered after reprocessing
The enrichment of 235U in reprocessed uranium (RepU) fuel has to be higher than in standard fuel, in order to compensate for the decrease in reactivity due to the presence of 234U and 236U. The use of RepU has a major impact on the choice of an enrichment process. If fuel is fabricated using only reprocessed uranium, the gas centrifuge process is better suited than the gaseous diffusion for enrichment of uranium, particularly because of the modular installations with relatively small capacity in gas centrifuge process. In addition, the modules are easier to cleanse of 234U and 236U than those of a gaseous diffusion plant.
Laser separation process
Isotopic separation of uranium can be achieved based on photo excitation principles (exciting the molecules using laser light). Such technologies have been named Atomic Vapor Laser Isotope Separation (AVLIS), Molecular Laser Isotope Separation (MLIS), and Separation of Isotopes by Laser Excitation (SILEX). They are based on the difference in the ionization energies of the isotopes of a given element. A laser beam illuminates vapour of uranium metal or uranium metal alloy and selectively ionizes the atoms of 235U, removing an electron from each and leaving them with a positive charge. 235U is then collected on negatively charged plates. Neutral 238U condenses on collectors on the roof of the separator.
Safety issues in refining/conversion and enrichment facilities
The safety issues related to refining/ conversion and enrichment facilities are documented in detail in the IAEA safety standards[6][7].
Generally in a refining/ conversion facility, only natural (or slightly enriched uranium from reprocessing) is processed. The radiotoxicity of this material is low, and thus the expected off-site radiological consequences following potential accidents are limited. However, in conversion facilities that process uranium with a 235U concentration of more than 1 % (using reprocessed uranium or scrap uranium pellets as feed material), criticality can also be a hazard.
The existing processes for natural uranium refining and conversion to hexafluoride usually give rise to no significant radiological hazards, and the safety problems associated with the handling of this material are essentially those of a conventional chemical industry dealing with toxic, corrosive, combustible and/or explosive chemicals such as hydrofluoric acid (HF) and fluorine (F2)[7]. Hazards of uranium hexafluoride (UF6) handling, storage and transportation are common to several stages of the nuclear fuel cycle, such as conversion, enrichment and fuel fabrication[8].
The conversion to UF6 of uranium recovered from the reprocessing of spent fuel from power reactors could give rise to an increase in radiological hazards (external exposure) associated with UF6 handling. This uranium product contains small quantities of plutonium, other actinides (232U) and fission products[7]. The latter may accumulate in some parts of the process, particularly in the hexafluoride production stage. Assessment and control of these hazards can be made by continuous monitoring of the radioactivity in the process vessels, although there is clearly no potential for a rapid increase in the activity levels.
The main credible hazard in an enrichment facility is a potential release of UF6. There is also a criticality hazard due to the handling of U with more than 1 % of 235U. As in conversion facilities there is an increased hazard of external radiation exposure in situations involving reprocessed U[7].
The refining/ conversion and enrichment process relies to a large extent on operator intervention and administrative controls to ensure safety, in addition to active and passive engineered safety measures.
In the following the main hazards, i.e. UF6 release, internal and external exposure, criticality, leaks of toxic chemicals, fire and explosions, and external hazards in a refining/ conversion and enrichment facility are discussed in more detail.
UF6 release
Failures of vessels, gaskets, valves, instruments or lines can result in either liquid or gaseous UF6 release. These failures can be caused by corrosion, mechanical damage, mal-operation of the system, or overheating of all or part of the system.
Four types of cylinders with capacities ranging from 2 to 12 t are commonly used for storage and transport of UF6, which is then in solid form. These cylinders need to be heated up for the transfer of UF6 into its gaseous form. Though UF6 is not in itself flammable, if a container were present in a fire, the container could explode by virtue of the internal stresses built up in the container and spread its contents over a wide area. The presence of oil or other (organic) impurities in storage cylinders or process equipment can lead to an exothermic reaction, which might give rise to a UF6 release[8]. The chemical toxicity of uranium in soluble form such as UF6 is more significant than its radiotoxicity.
At room temperature, at which it is handled and stored, UF6 is a colourless, crystalline solid with a significant but low vapour pressure. When heated at atmospheric pressure to facilitate transfer, the crystals sublime without melting and the vapour pressure reaches 760 mm Hg at a temperature of about 56°C. At higher pressures, the crystals will melt, at a temperature of about 64oC and this melting is accompanied by a very substantial increase in specific volume.
Uranium hexafluoride is a highly reactive substance. It reacts chemically with water, forming soluble reaction products with most organic compounds and with many metals. Its reactivity with most saturated fluorocarbons is very low. It does not react with oxygen, nitrogen, or dry air.
The prime hazard following a UF6 release arises from the reaction between UF6 and moisture, which is normally present in the atmosphere producing two toxic substances hydrofluoric acid (HF) and uranyl fluoride (UO2F2) according to the equation:
With gaseous UF6, this reaction proceeds rapidly liberating some heat and is accompanied by a substantial volume increase at atmospheric pressure. Both UO2F2 and HF are toxic. Deposition of UO2F2 from a cloud formed following a release to the outside of the facility could result in the contamination of agricultural crops and grassland. The rate at which deposition will occur and hence the contamination contours will be very dependent on atmospheric conditions at the time of release. Calculation of the dispersion of toxic material following a UF6 release is complicated by virtue of the high density levels of some of the products and the chemical reactions which occur.
UF6 leakage needs to be restricted to less than 0.2 mg/m3 (= chemical toxicity limit for natural U and up to 2.5 % enrichment).
There have been several accidents involving UF6. As early as 1944, in the United States, a weld ruptured on an 8 ft (~240 cm) long cylinder containing gaseous natural UF6 that was being heated by steam. An estimated 400 lb (~181 kg) of UF6 was released, which reacted with steam from the process and created HF and uranyl fluoride. This accident resulted in two deaths from HF inhalation and three individuals seriously injured from both HF inhalation and uranium toxicity. Another UF6 accident involving a cylinder rupture occurred at a commercial uranium conversion facility (Sequoya Fuels Corp., USA) in 1986. The accident occurred when an over-loaded shipping cylinder was reheated to remove an excess of UF6. The cylinder ruptured, releasing a dense cloud of UF6 and its reaction products. This accident resulted in the death of one individual from HF inhalation.
Internal and external radiation exposure
Inhalation of uranium compounds leads to internal radiation exposure. Depending on the solubility and diameters of particulates, inhalations of different uranium compounds give different doses. Ref[9] provides effective dose coefficients for inhaled particulates for workers which can be used to estimate the activity and mass of uranium that give 20 mSv on inhalation. The result of such estimations for the case of activity median aerodynamic diameters of particulates equal to 5 μm is presented in Table 1.
| Class (depending on solubility) | Arctivity* (Bq) | Mass (mg) | |
|---|---|---|---|
| natural U | 3.5% enriched U | ||
| S Class – UO2, U3O8, U | 3509 | 140 | 33 |
| M Class – UO3, UF4, MDU, ADU | 12500 | 503 | 115 |
| F Class – UF6, UO2F2, UO2(NO3)2 | 34482 | 1379 | 316 |
Note:
* – activity is subjected to small variations with respect to enrichment but for the purpose of INPRO assessment can be considered as independent of enrichment.
The natural and enriched uranium but also the depleted uranium in a UF6 cylinder emits neutron and gamma radiation from thorium and its progenies. In case of reprocessed uranium in addition to the presence of Pu and fission products the build-up of 232U can lead to radiation exposure.
In the proximity of cylinders carrying depleted uranium, up to 20 % of the radiation exposure can be due to the neutron radiation. In the proximity of cylinders carrying enriched uranium, up to 70 % of the radiation exposure can be due to neutron radiation.
Criticality
Depending upon the concentration, mass and enrichment, degree of moderation and reflection, and presence of appropriate neutron absorbers, material with enriched U can attain criticality in some geometries. Hence safe geometries need to be ensured in the facility based on specific criticality analyses. Criticality is not possible with gaseous UF6. At low enrichments of less than 1 % of 235U, even liquids with moderation do not go critical. For higher enrichments, moderation is important. Typically at 7 % enrichment, the H/U atom ratio needs to be kept below 0.38 (see also Section 2.2 of NFCF). Criticality monitoring and alarm systems are mandatory in such an NFCF.
Containment of radioactive material and/or toxic chemicals
Inside the facility, leaks of radioactive material[7] such as uranium solutions and powders, gaseous or liquid UF6 and toxic chemicals such as HF, F2, NH3, ClF3 from systems consisting of vessels, pumps, valves and pipes can lead to dispersion (and unnecessary generation of waste). Leaks of hydrogenous fluids (water, oil, etc.) leading to flooding can adversely affect criticality safety parameters such as reflection and moderation. Leaks of flammable gases (e.g. H2) or liquids can lead to explosions and/or fires.
Leak detection systems are expected to be deployed in locations where leaks could occur. Vessels containing significant amounts of nuclear material in solution form need to be equipped with level detectors and alarms to prevent overfilling and with secondary containment features such as bunds or drip trays of appropriate capacity and configuration to ensure criticality safety (see also Section 2.2 of NFCF).
To prevent a release of radioactive material and/or toxic chemical to the outside of the plant several barriers (combinations of ‘static’ and ‘dynamic’ containments) are necessary: The first barrier is the casing of the equipment (e.g. wall of a cylinder, vessel or pipe), and the second barrier is the building wall of the facility. Additionally, dynamic containments are to be provided by ventilation systems in process equipment and in the working area of the facility.
Fire and explosions
Detailed recommendations on the consideration of fire and explosions in refining/ conversion facilities and enrichment facilities design are provided in Ref[7]. Fire in a refining/ conversion and enrichment facility can occur due to the existence of materials such as anhydrous ammonia (explosive and flammable), nitric acid (ignition if in contact with organic materials) and hydrogen. A fire can lead to the dispersion of radioactive and/or toxic material by breaching the containment barriers or may even cause a criticality accident (see also Section 2.4 of NFCF).
External hazards
Detailed recommendations on the consideration of initiating events associated with external hazards are provided in Ref[7]. Conversion facilities and enrichment facilities need to be designed for all credible external hazards (see Section 2.1 of NFCF), e.g. the design needs to consider an earthquake to ensure that the integrity of the confinement is assured (especially for UF6 and HF) and no change in criticality parameters such as geometry and moderation is induced (see also Section 2 of NFCF).
See also
- NFCF
- Mining and milling of uranium and thorium
- Uranium oxide and MOX fuel fabrication
- Reprocessing of spent nuclear fuel
- Storage of spent nuclear fuel
[ + ] Assessment Methodology | |||||
|---|---|---|---|---|---|
|
|||||
References
- ↑ OECD/NUCLEAR ENERGY AGENCY (NEA), The Safety of the Nuclear Fuel Cycle, Third Edition, NEA No.3588, OECD/NEA, Paris (2005).
- ↑ OLIVER, A., OZBERK, E., Conversion of Natural Uranium, Ch 11 In Hore-Lacy, I. (Ed.), Uranium for Nuclear Power: Resources, Mining and Transformation to Fuel. Woodhead Publishing, Cambridge (2016)
- ↑ HERIOT, I.D., Uranium Enrichment by Centrifuge, Report EUR-11486, Commission of the European Communities, Brussels (1988).
- ↑ KEHOE, R.B., The Enriching Troika, a History of URENCO to the Year 2000, Urenco, Marlow, UK, (2002).
- ↑ HARDING, P., Uranium Enrichment, Ch 12 In Hore-Lacy, I. (Ed.), Uranium for Nuclear Power: Resources, Mining and Transformation to Fuel. Woodhead Publishing, Cambridge (2016)
- ↑ INTERNATIONAL ATOMIC ENERGY AGENCY, Safety of Nuclear Fuel Cycle Facilities, IAEA Safety Standards, Specific Safety Requirements No. SSR-4, IAEA, Vienna (2017).
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 INTERNATIONAL ATOMIC ENERGY AGENCY, Safety of Conversion Facilities and Uranium Enrichment Facilities, IAEA Safety Standards, Specific Safety Guide No. SSG-5, IAEA, Vienna (2010).
- ↑ 8.0 8.1 INTERNATIONAL ATOMIC ENERGY AGENCY, Manual on Safe Production, Transport, Handling and Storage of Uranium Hexafluoride, IAEA-TECDOC-771, IAEA, Vienna (1994).
- ↑ INTERNATIONAL COMMISSION ON RADIOLOGICAL PROTECTION, Compendium of Dose Coefficients Based on ICRP Publication 60, ICRP Publication 119, Elsevier (2012).